Understanding Electrochemical Principles: The Science Behind Many Modern Sensors
In the modern world, sensors play a critical role in industries ranging from healthcare and environmental monitoring to automotive systems and smart infrastructure. Among the various sensing technologies available today, electrochemical sensors have become increasingly prominent due to their high sensitivity, selectivity, and relatively low cost. But what makes electrochemical sensors so effective? The answer lies in the fundamental scientific concept they are built upon—electrochemical principles.
Electrochemistry is a branch of chemistry that deals with the relationship between electrical energy and chemical changes. While it may sound abstract, electrochemical reactions are deeply ingrained in everyday life, powering batteries, enabling corrosion protection, and even driving metabolic processes in the human body. In sensor applications, electrochemical principles are harnessed to detect and quantify specific chemical species such as gases, ions, or biomolecules.
This article aims to demystify electrochemical principles, explain how they are applied in sensor technologies, and highlight their importance in the development of reliable, real-time monitoring solutions.
What Is Electrochemistry?
Definition
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is what creates electric current. Electrochemical reactions typically occur at the interface between an electrode (a solid conductor) and an electrolyte (a liquid or solid that contains free ions and conducts electricity).
Basic Concepts
To understand electrochemical principles, it's essential to grasp the following key concepts:
-
Redox Reactions: Short for "reduction-oxidation" reactions, these involve the transfer of electrons from one substance to another.
-
Oxidation is the loss of electrons.
-
Reduction is the gain of electrons.
-
-
Electrode: A conductor through which electricity enters or leaves an electrochemical system.
-
Anode: Electrode where oxidation occurs.
-
Cathode: Electrode where reduction occurs.
-
-
Electrolyte: A medium (often a liquid solution) containing ions that can move to carry electric current.
-
Cell Potential (Voltage): The difference in electric potential between two electrodes; it's a measure of the tendency of electrons to flow from one electrode to the other.
Types of Electrochemical Cells
Electrochemical cells are systems in which electrochemical reactions occur. They are broadly divided into two categories:
1. Galvanic (Voltaic) Cells
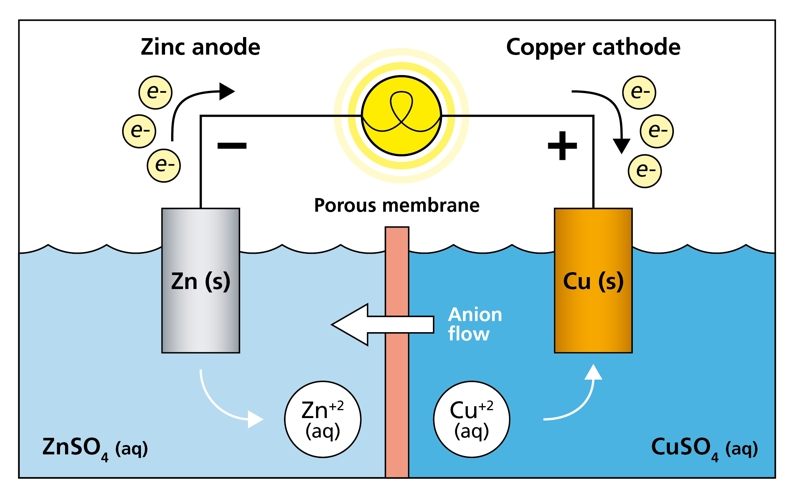
These cells generate electrical energy from spontaneous chemical reactions. Common examples include batteries like AA cells or lithium-ion batteries.
2. Electrolytic Cells
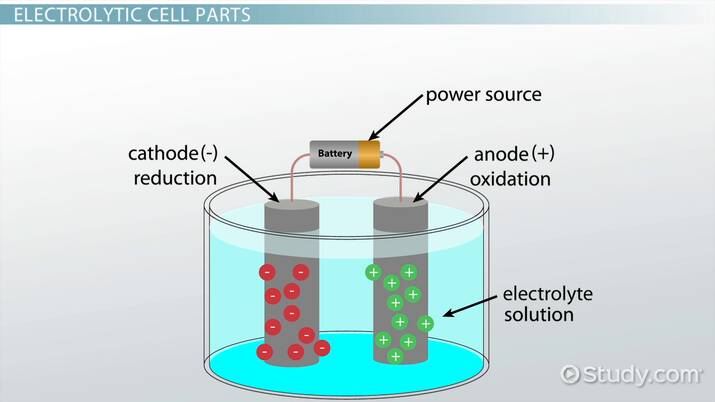
These cells consume electrical energy to drive non-spontaneous chemical reactions. Electrolysis of water (splitting water into hydrogen and oxygen) is a classic example.
For sensor applications, both types can be relevant, but galvanic and amperometric systems are more commonly used.
How Electrochemical Principles Apply to Sensors
Electrochemical sensors operate by measuring an electrical signal that correlates with the concentration of a target analyte. Here’s a breakdown of how the system typically works:
Components of an Electrochemical Sensor
-
Working Electrode: The site where the analyte undergoes oxidation or reduction.
-
Counter Electrode: Completes the circuit by allowing current to flow.
-
Reference Electrode: Provides a stable voltage against which the working electrode potential is measured.
-
Electrolyte: Facilitates the transfer of ions between electrodes.
Working Principle
-
The analyte (e.g., a gas like CO or NO₂) diffuses through a membrane and reaches the working electrode.
-
A redox reaction occurs, generating or consuming electrons.
-
This flow of electrons creates an electrical current or potential.
-
The sensor’s electronics interpret this signal and convert it into a concentration value.
Winsen Electrochemical Sensor

EC Hazardous Toxic Gas Detection Sensor Module ZE03
- CO,O2,NH3,H2S,NO2,O3,SO2, CL2,HF,H2,PH3,HCL, etc.
- See manual
- Read More
Applications of Electrochemical Sensors
1. Environmental Monitoring
-
Detect pollutants like ozone (O₃), nitrogen dioxide (NO₂), sulfur dioxide (SO₂), and carbon monoxide (CO).
-
Monitor air and water quality.
2. Industrial Safety
-
Gas leak detection in chemical plants, oil refineries, and confined spaces.
-
Monitoring combustible or toxic gases to prevent accidents.
3. Medical Diagnostics
-
Glucose sensors for diabetes management.
-
Lactate sensors for metabolic monitoring.
-
Electrochemical biosensors for detecting pathogens or biomarkers.
4. Automotive Applications
-
Monitoring exhaust gases to meet emission standards.
-
Cabin air quality sensors.
5. Smart Infrastructure
-
Air quality monitoring in smart buildings.
-
Integration with HVAC systems for ventilation control.
Advantages of Electrochemical Sensors
-
High Sensitivity: Capable of detecting trace levels of analytes.
-
Good Selectivity: Target-specific redox reactions reduce interference.
-
Low Power Consumption: Ideal for battery-powered or portable devices.
-
Compact Size: Suitable for integration into IoT and wearable systems.
-
Cost-Effective: Low production cost compared to other sensor types.
Challenges and Limitations
Despite their advantages, electrochemical sensors also face certain challenges:
-
Limited Lifespan: Electrodes can degrade over time.
-
Cross-Sensitivity: Some sensors may respond to multiple gases.
-
Calibration Requirements: Regular calibration is needed for accurate results.
-
Environmental Effects: Humidity and temperature can influence sensor performance.
Manufacturers address these issues through sensor design improvements, signal processing algorithms, and environmental compensation techniques.
Innovations and Future Trends
The field of electrochemical sensing is rapidly evolving. Some notable trends include:
1. Miniaturization and Integration
-
Development of microelectromechanical systems (MEMS)-based sensors.
-
Integration with smartphones and wearable devices.
2. Multi-Gas Sensing Platforms
-
Combining multiple sensors in a single package.
-
Using machine learning for pattern recognition and enhanced selectivity.
3. Flexible and Printed Sensors
-
Using printable materials for low-cost, disposable sensor strips.
-
Applications in healthcare and food safety.
4. Electrochemical Biosensors
-
Integration with biological recognition elements like enzymes, antibodies, or DNA.
-
Rapid detection of viruses, bacteria, or toxins.
Conclusion
Electrochemical principles form the scientific foundation of many modern sensor technologies. By converting chemical reactions into electrical signals, electrochemical sensors provide a versatile and powerful tool for detecting gases, ions, and biomolecules across a wide range of applications.
Understanding these principles not only enhances our appreciation of sensor performance but also helps engineers and developers design more effective, reliable, and energy-efficient systems. As technology advances, electrochemical sensors will continue to play a vital role in building a safer, smarter, and more sustainable future.