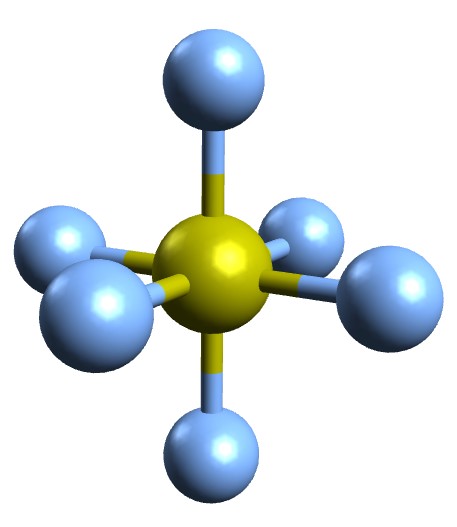
Sulfur Hexafluoride (SF₆): Properties, Applications, Hazards, and Environmental Impact
Sulfur hexafluoride (SF₆) is a colorless, odorless, non-flammable, and non-toxic gas that has become an essential dielectric medium in the power industry and other high-voltage applications. Known for its excellent electrical insulation and arc-quenching properties, SF₆ plays a pivotal role in gas-insulated switchgear (GIS), circuit breakers, particle accelerators, and medical imaging systems.
Despite its usefulness, SF₆ has come under scrutiny due to its potent greenhouse gas (GHG) properties and long atmospheric lifespan. This article explores SF₆’s chemical and physical characteristics, industrial applications, handling and safety, environmental concerns, and alternatives.
Basic Properties of SF₆
| Property | Value |
|---|---|
| Chemical Formula | SF₆ |
| Molecular Weight | 146.06 g/mol |
| Appearance | Colorless gas |
| Odor | Odorless |
| Boiling Point | -64°C (-83.2°F) |
| Melting Point | -50.8°C (-59.4°F) |
| Density (gas at 25°C) | 6.17 kg/m³ |
| Solubility in Water | Slightly soluble |
| Dielectric Strength | About 2.5 times greater than air |
| GWP (Global Warming Potential) | 23,500 (over 100 years) |
| Atmospheric Lifetime | ~3,200 years |
SF₆ is a synthetic gas, meaning it is not naturally found in the Earth’s atmosphere. It is extremely stable chemically, which contributes to both its utility and its environmental persistence.
Physical and Chemical Characteristics
Stability
SF₆ is inert under normal conditions. It is non-reactive with many materials and does not support combustion or sustain life. These qualities make it ideal for use in sealed environments.
Dielectric Properties
SF₆ has an exceptionally high dielectric strength, which is one of the primary reasons it is used in high-voltage switchgear. It can suppress electrical discharge and arc formation better than air or nitrogen.
Arc Quenching
When SF₆ is exposed to an electric arc, it dissociates and absorbs energy, helping to quickly quench the arc and prevent electrical faults.
Industrial Applications
Electrical Industry
- Gas-Insulated Switchgear (GIS): SF₆ is used to insulate and protect high-voltage components.
- Circuit Breakers: The gas interrupts high-voltage arcs during circuit disconnection.
- Transformers and Bushings: Insulates live parts, preventing short circuits and fires.
- Gas-insulated transmission lines (GIL): SF₆ enables compact and efficient high-voltage transmission.
Medical Applications
- Ophthalmology: SF₆ is used in retinal detachment surgeries as a tamponade gas.
- MRI and Radiology: Acts as a contrast agent in certain imaging techniques.
Electronics and Semiconductor Manufacturing
- Plasma Etching: SF₆ is used in reactive ion etching (RIE) of silicon-based materials.
- Dielectric Testing: Used as a calibrant gas for electrical testing.
Particle Physics
- Particle Accelerators and Detectors: SF₆ is used as a gaseous insulator in particle detectors and high-voltage accelerators.
Handling and Storage
Storage Conditions
- Store SF₆ in high-pressure cylinders or tanks in well-ventilated areas.
- Keep away from open flames or high temperatures to avoid decomposition.
Transportation
- SF₆ is classified as a non-flammable compressed gas under most transport regulations.
- Cylinders must meet regulatory standards for gas containment and pressure ratings.
Decomposition Products
When SF₆ is exposed to electrical discharges, it can decompose into toxic byproducts, including:
- Sulfur dioxide (SO₂)
- Hydrogen fluoride (HF)
- Disulfur decafluoride (S₂F₁₀)
These compounds are corrosive and harmful if inhaled, and appropriate detectors and filtration systems are required to ensure safety.
Safety and Toxicity
Health Hazards
- SF₆ is not toxic in its pure form, but it can act as an asphyxiant by displacing oxygen in confined spaces.
- Decomposition products, however, can be highly toxic and corrosive.
Protective Measures
- Use gas detectors for both SF₆ and its breakdown products.
- Provide adequate ventilation in enclosed areas.
- Use personal protective equipment (PPE), including gloves and eye protection when handling cylinders or systems containing SF₆.
Exposure Limits
- There are no OSHA permissible exposure limits (PELs) for SF₆ itself, but NIOSH and manufacturers recommend avoiding prolonged exposure and ensuring oxygen levels above 19.5%.
Sulfur Hexafluoride Sensor
Environmental Impact
SF₆ is among the most potent greenhouse gases known.
Global Warming Potential (GWP)
- One kilogram of SF₆ is equivalent to 23,500 kg of CO₂ over a 100-year time frame.
- Its atmospheric residence time exceeds 3,000 years.
Emissions and Leakage
Leaks can occur during:
- Manufacturing
- Maintenance and servicing of switchgear
- Cylinder refilling and disposal
Regulatory Frameworks
- Kyoto Protocol: SF₆ is listed among six major GHGs.
- EU F-Gas Regulation: Places strict limits on use and reporting of SF₆ in Europe.
- EPA Greenhouse Gas Reporting Program (GHGRP): Requires tracking and reporting SF₆ emissions in the U.S.
Detection and Monitoring
Given its environmental risk, SF₆ leak detection is a priority in the industry.
Detection Technologies
- Infrared (IR) Spectroscopy: Detects SF₆ using its unique IR absorption profile.
- Gas Chromatography (GC): Lab-based, highly accurate detection method.
- Ultrasonic Leak Detectors: Identify high-pressure gas escaping from equipment.
- Electrochemical Sensors: Effective for detecting decomposition products like SO₂ and HF.
Monitoring Protocols
- Regular equipment inspection
- Portable and fixed gas detection systems
- Smart grid diagnostics with automated leak tracking
Alternatives to SF₆
Due to environmental concerns, several alternatives are being explored:
| Alternative | Description |
|---|---|
| g³ (Green Gas for Grid) | A mix of fluoronitrile and CO₂; similar dielectric strength to SF₆ |
| Dry Air | Used in medium-voltage equipment; non-toxic, zero GWP |
| CO₂ + O₂ or N₂ mixtures | Used in low to medium voltage applications |
| Vacuum Interruption | In circuit breakers; suitable for MV systems |
Challenges
- Cost: New technologies can be more expensive.
- Size and complexity: Alternative systems may require larger equipment.
- Performance: Must match the reliability and dielectric properties of SF₆.
Recycling and Recovery
To mitigate emissions and reduce environmental impact, SF₆ recovery and recycling systems are widely used:
Recovery Units
Portable or stationary units recover SF₆ gas from GIS or breakers during maintenance.
Recycling Process
- Remove particulates and moisture
- Use filters and scrubbers to remove toxic byproducts
- Store and reuse the purified gas
Disposal
If not recyclable, SF₆ must be destroyed using high-temperature incineration in certified facilities.
Conclusion
Sulfur hexafluoride (SF₆) is a critical component in modern electrical infrastructure, enabling compact, efficient, and reliable high-voltage systems. Its unmatched dielectric and arc-extinguishing properties have cemented its place in global industries ranging from energy to healthcare.
However, the environmental cost of SF₆ cannot be ignored. With its extremely high GWP and long atmospheric life, efforts to monitor, recycle, and replace SF₆ are essential. Innovations in alternative gases, better leak detection, and tighter regulations are helping to reduce its impact while maintaining system reliability.
As industries transition to sustainable practices, understanding and managing SF₆ responsibly will remain a top priority.
References
- Intergovernmental Panel on Climate Change (IPCC)
- U.S. Environmental Protection Agency (EPA)
- International Electrotechnical Commission (IEC)
- IEEE Standards on GIS and SF₆ Handling
- Manufacturers’ Safety Data Sheets (MSDS)