Electrochemical Hydrogen Sensors: Principles, Applications, and Advantages
Introduction
As hydrogen continues to gain popularity as a clean and renewable energy source, the need for accurate, reliable, and cost-effective hydrogen detection becomes critical. One of the most effective technologies for this task is the electrochemical hydrogen sensor. These sensors offer high sensitivity, selectivity, and are widely used in industrial safety, fuel cell systems, hydrogen storage facilities, and environmental monitoring.
This article will explore the working principles, design, advantages, limitations, and typical applications of electrochemical hydrogen sensors, along with insights into how they compare with other hydrogen detection technologies.
What Is an Electrochemical Hydrogen Sensor?
An electrochemical hydrogen sensor is a type of gas sensor that detects hydrogen gas (H₂) through an electrochemical reaction. When hydrogen molecules come into contact with the sensor's surface, they undergo oxidation or reduction reactions, generating an electrical signal proportional to the hydrogen concentration in the environment.
These sensors are particularly known for:
- High sensitivity (down to parts per million, ppm)
- Strong selectivity to hydrogen
- Low power consumption
- Compact design
How Electrochemical Hydrogen Sensors Work
Electrochemical sensors typically consist of the following components:
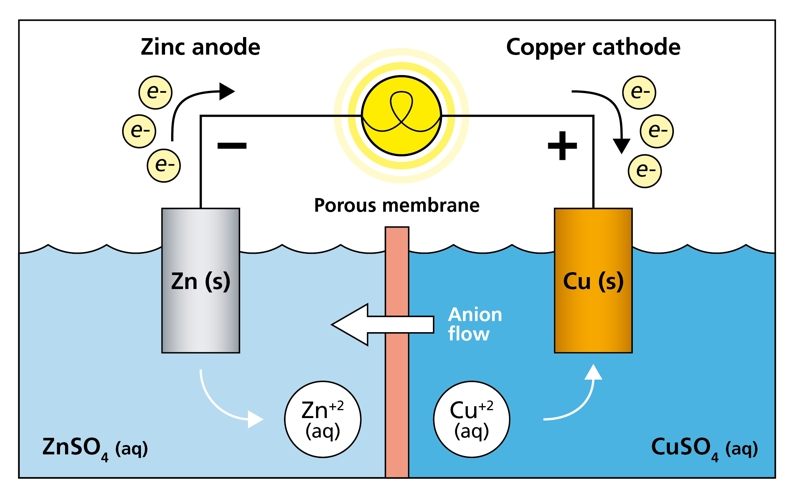
1. Working Electrode
This is where the hydrogen gas reacts. The electrode is typically made of platinum or palladium, materials that catalyze hydrogen oxidation reactions efficiently.
2. Counter Electrode
It completes the circuit by facilitating a corresponding reduction reaction.
3. Reference Electrode
Used to stabilize and control the potential at the working electrode, ensuring consistent measurements.
4. Electrolyte
A medium (often liquid or solid polymer) that allows ion transport between electrodes during the redox reactions.
Working Principle
When H₂ gas enters the sensor:
- Hydrogen molecules are oxidized at the working electrode, producing protons (H⁺) and electrons (e⁻).
- The protons migrate through the electrolyte to the counter electrode.
- The resulting current is proportional to the hydrogen concentration in the surrounding environment.
The output signal is then processed and calibrated to display the exact concentration of hydrogen gas.
Winsen Electrochemical Hydrogen Sensors

EC Hazardous Toxic Gas Detection Sensor Module ZE03
- CO,O2,NH3,H2S,NO2,O3,SO2, CL2,HF,H2,PH3,HCL, etc.
- See manual
- Read More
Applications of Electrochemical Hydrogen Sensors
1. Hydrogen Fuel Cell Vehicles
Electrochemical sensors monitor hydrogen leaks in storage tanks and fuel lines to ensure passenger safety.
2. Industrial Safety
Used in hydrogen production plants, laboratories, and refineries to prevent explosions due to undetected leaks.
3. Battery Monitoring
Hydrogen can evolve in sealed lead-acid and lithium batteries. Sensors detect buildup in enclosures to avoid fire hazards.
4. Power Plants
Especially in nuclear and thermal power stations, hydrogen is often used as a cooling agent for generators.
5. Hydrogen Storage Facilities
Continuous monitoring in hydrogen storage or filling stations ensures safety and compliance with regulations.
Advantages of Electrochemical Hydrogen Sensors
High Sensitivity
Can detect hydrogen concentrations as low as a few ppm.
High Selectivity
Minimal interference from other gases like CO₂, CO, or CH₄.
Low Power Consumption
Ideal for portable and remote sensing applications.
Compact and Lightweight
Suited for embedded applications in fuel cells and vehicles.
Linear Output
Facilitates easy signal processing and calibration.
Limitations of Electrochemical Hydrogen Sensors
Despite their advantages, there are some challenges:
- Limited Lifetime: Typically 1–2 years depending on usage and environment.
- Temperature/Humidity Sensitivity: Extreme conditions may affect performance.
- Cross-Sensitivity: While low, certain gases (e.g., strong oxidizers) may still cause interference.
- Calibration Requirement: Periodic calibration is necessary for accurate readings.
Electrochemical vs. Other Hydrogen Sensor Technologies
| Parameter | Electrochemical | Semiconductor (MOS) | Catalytic Combustion | Thermal Conductivity |
|---|---|---|---|---|
| Sensitivity | High | Moderate | Low–Moderate | Low |
| Selectivity | High | Low | Moderate | Low |
| Power Consumption | Low | High | High | Moderate |
| Response Time | Fast | Moderate | Slow–Moderate | Moderate |
| Cost | Moderate | Low | Low | Low |
| Typical Use Case | Safety systems, labs | Low-cost H₂ detection | Explosion-proof systems | Rough concentration checks |
Best Practices for Using Electrochemical Hydrogen Sensors
- Calibration: Perform regular calibration (every 6–12 months) using certified gas standards.
- Avoid Contaminants: Exposure to silicone, oil vapors, or solvents can reduce sensor lifespan.
- Install Properly: Mount in areas with good airflow; avoid locations with excessive humidity or temperature extremes.
- Monitor Drift: Log long-term sensor data to observe potential performance drift or failure.
Maintenance and Lifetime
Electrochemical sensors have a finite lifespan, typically 1–3 years, influenced by:
- Gas exposure level and frequency
- Operating temperature and humidity
- Storage and usage conditions
To ensure maximum service life:
- Keep sensors in their original sealed packaging before installation.
- Avoid unnecessary exposure to high concentrations of hydrogen during non-operational periods.
- Use proper filtering if exposed to dust or aerosols.
FAQs About Electrochemical Hydrogen Sensors
Q1: How often should electrochemical hydrogen sensors be replaced?
Most sensors have a lifespan of 12 to 36 months. Replacement intervals depend on the operating environment and usage.
Q2: Are electrochemical sensors suitable for outdoor use?
Yes, but they require protection from rain, dust, and temperature extremes. Enclosures and filters are recommended.
Q3: What is the warm-up time for electrochemical sensors?
Usually less than 60 seconds. However, optimal accuracy may take a few minutes after startup.
Q4: Can they detect hydrogen in mixtures with other gases?
Yes, but performance depends on the concentration and interference of other gases. High selectivity helps reduce false positives.
Conclusion
Electrochemical hydrogen sensors play a critical role in the global shift toward hydrogen-based energy solutions. With their high sensitivity, low power consumption, and strong selectivity, they are ideal for a broad range of industrial and environmental applications. As safety standards tighten and hydrogen adoption expands, these sensors will remain indispensable components in ensuring safe, efficient, and sustainable hydrogen use.