LEL Gas Chart (Lower Explosive Limit): Complete Guide + Common Gas Table
A LEL gas chart is a quick-reference table that lists the Lower Explosive Limit (LEL) and Upper Explosive Limit (UEL) for common flammable gases and vapors—typically shown as percent by volume in air (% v/v). Safety teams use it to set gas detector alarms, evaluate hot-work risk, and plan ventilation. OEM designers use it to choose the right combustible gas sensor and calibrations.
Core definitions (LEL vs UEL):
- LEL / LFL (Lower Explosive/Flammable Limit): the minimum concentration of fuel in air that can ignite.
- UEL / UFL (Upper Explosive/Flammable Limit): the maximum concentration of fuel in air that can ignite.
- Between them is the flammable range; below LEL is “too lean,” above UEL is “too rich” (but can become dangerous as it dilutes).
More About LEL and UEL: LEL and UEL: The Complete Guide to Explosive Limits, %LEL, and Gas Detection
How to Read an LEL Gas Chart
Most charts show:
- Gas/Vapor name + chemical formula
- LEL (% v/v) and UEL (% v/v)
- Sometimes notes about temperature/pressure or measurement conditions
Important: LEL/UEL values can vary with test method and conditions. Many reference tables explicitly note that explosive limits are valid only under the conditions they were determined and that the flammability range often expands as temperature, pressure, and vessel diameter increase.
LEL Gas Chart for Common Gases and Vapors (Quick Table)
Below is a practical “most-used” LEL/UEL chart for gas detection and site safety. Values are % by volume in air.
Reference note: Numbers are compiled from widely used explosive-limit tables. Always confirm with your SDS and local code requirements for final safety decisions.
| Gas / Vapor | Formula | LEL (% v/v) | UEL (% v/v) |
|---|---|---|---|
| Methane (Natural Gas) | CH₄ | 5.0 | 15.0 |
| Propane (LPG) | C₃H₈ | 2.1 | 10.1 |
| n-Butane | C₄H₁₀ | 1.86 | 8.41 |
| Isobutane | C₄H₁₀ | ~1.8 | ~8.4–9.6 |
| Hydrogen | H₂ | 4.0 | 75.0 |
| Carbon Monoxide | CO | 12.0–12.5 | ~74–75 |
| Hydrogen Sulfide | H₂S | ~4.0–4.3 | ~44–46 |
| Ethane | C₂H₆ | 3.0 | 12.4 |
| Ethylene | C₂H₄ | 2.75 | 28.6 |
| Propylene | C₃H₆ | 2.0 | 11.1 |
| Acetylene | C₂H₂ | 2.5 | 80–100 |
| Ammonia* | NH₃ | 15.0 | 27–28 |
| Benzene | C₆H₆ | ~1.3–1.35 | ~6.65–7.9 |
| Toluene | C₇H₈ | 1.27 | 6.75 |
| Xylene (mixed) | C₈H₁₀ | ~1.0 | ~6.0 |
| Styrene | C₈H₈ | 1.1 | 6.1 |
| Acetone | C₃H₆O | 2.6 | 12.8–13.0 |
| Methanol | CH₃OH | 6.7 | 36.0 |
| Ethanol | C₂H₅OH | 3.3 | 19.0 |
| Isopropanol | C₃H₈O | ~2.0–2.2 | ~12 (varies) |
| Ethyl acetate | C₄H₈O₂ | 2.0 | 12.0 |
| Ethylbenzene | C₈H₁₀ | 1.0 | 7.1 |
| Diethyl ether | C₄H₁₀O | 1.9 | 36–48 |
| Hexane | C₆H₁₄ | ~1.2–1.25 | ~7.0–7.4 |
| Heptane | C₇H₁₆ | ~1.0–1.1 | ~6.0–6.7 |
| Pentane | C₅H₁₂ | 1.4 | 7.8 |
| Gasoline (vapors) | — | ~1.4 | ~7.6 |
| Diesel fuel (vapors) | — | ~0.6 | ~7.5 |
| Kerosene / Jet fuel vapors | — | ~0.7 | ~5 |
*Ammonia is often treated primarily as toxic, but it does have flammable limits.
Sources for these tables include well-known compiled references and engineering tables.
%LEL Explained (And How to Convert %LEL to Gas Volume %)
Most combustible gas detectors read %LEL (percent of the LEL).
Conversion formula (gas-specific):
Volume% = (%LEL ÷ 100) × LEL(vol%)
Example (methane): LEL = 5% vol
- 10% LEL ≈ 0.10 × 5% = 0.5% vol methane
- 25% LEL ≈ 0.25 × 5% = 1.25% vol methane
Reverse conversion (% vol → %LEL):
%LEL = vol% × (100 / LEL vol%)
Why “10% LEL” Is a Big Deal (Confined Spaces & Hot Work)
Many high-ranking safety resources highlight 10% LEL because OSHA defines a hazardous atmosphere in permit-required confined spaces as:
- Flammable gas/vapor/mist in excess of 10% of its LFL
OSHA shipyard guidance also adds a critical nuance:
- ≥10% LEL is hazardous in confined spaces, but <10% LEL is not necessarily safe (it can signal ongoing vapor release and conditions may worsen).
Practical implication: 10% LEL is often used as an early action threshold (ventilation, investigation, stop-work triggers), but safety decisions must consider trend, ventilation, work activity, and oxygen conditions.
What Changes LEL/UEL
Explosive limits are influenced by conditions—many references note the flammable range can expand with:
- Higher temperature
- Higher pressure
- Larger enclosure/test diameter
- Oxygen enrichment (broader flammable range, faster burning)
This is why best practice is:
- Treat charts as baseline references
- Verify with SDS data and applicable standards
- Measure on-site with properly calibrated instruments
Mixed Gases: The Le Chatelier LEL Mixing Rule
Real sites often have mixtures (e.g., methane + propane + solvent vapors). A widely used approximation for the mixture LEL is Le Chatelier’s mixing rule, commonly referenced in safety engineering literature:
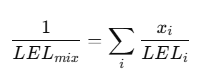
Where (x_i) is the volume fraction of component (i) in the fuel mixture.
Use case: estimating alarms and risk when multiple fuels may be present.
Limitations: it’s an approximation; always validate for critical safety scenarios.
Common combustible sensing technologies
- Catalytic (pellistor): strong for many combustible gases; needs oxygen; can be poisoned by certain compounds
- NDIR infrared: excellent for many hydrocarbons; often more resistant to poisoning; typically not for hydrogen
- MOS semiconductor: compact/cost-effective; may require stronger compensation for environment/cross-sensitivity depending on application
Standards to know
IEC performance standards for gas detectors have evolved; IEC notes that IEC 60079-29-1 has been replaced, and the newer IEC 60079-29-0:2025 covers general requirements and test methods across gas detection equipment categories.
Winsen OEM Support
If you’re building gas alarms, HVAC safety controls, industrial transmitters, or IoT safety gateways, a dependable combustible sensor helps you hit performance targets and improve product differentiation.
Winsen supports combustible gas sensing solutions for OEM integration (multiple sensing principles, integration formats, and engineering support). If you tell us:
- target gas (CH₄ / LPG / H₂ / mixed)
- measurement range (%LEL)
- environment (temperature, humidity, solvents, dust)
- interface needs (analog / UART / RS485 / relay)
We can recommend a sensor approach and support customization + selection + integration.
Catalytic Combustion (Pellistor) Sensors

MR007 CH4 Methane C3H8 Propane Gas Sensor
- CH4 methane C3H8 propane, combustible gas, natural gas, coal gas, LPG gas
- 0~100 LEL
- Read More

ZC13 Methane CH4 Sensor Module for Home Gas Safety
- methane CH4, natural gas, flammable gas
- 1%-25%LEL,Resolution100ppm
- Read More

MC119 Catalytic Flammable Gas Sensor
- hydrogen, acetylene, gasoline, VOC such as alcohol, ketone, benzene.
- 0-100%LEL Anti-explosion Mark:ExdibⅠ
- Read More

ZC08-CH4 Methane Sensor Module for Home Natural Gas Leakage
- methane CH4, natural gas, flammable gas
- 1%-20%LEL,Resolution100ppm
- Read More
Infrared (NDIR) Combustible Gas Sensors
MOS (Semiconductor) Sensors

MPn-4C CH4 Methane Flammable Gas Sensor
- CH4, Methane, Natural gas, marsh gas
- 300~10000ppm (methane, natural gas)
- Read More
TDLAS Laser Methane(CH4) Sensor
Explore Winsen Combustible Sensor Options: https://www.winsen-sensor.com/combusitable-sensor/
FAQs
What is an LEL gas chart used for?
To quickly compare explosive limits of common gases/vapors, set %LEL alarm thresholds, and support ventilation and hot-work/confined-space decisions.
Is above UEL “safe”?
Not necessarily. A rich mixture may not ignite immediately, but as it mixes with air it can pass back through the flammable range.
Why do detectors alarm at 10% LEL?
OSHA uses thresholds around 10% LFL/LEL for defining hazardous flammable atmospheres in specific confined-space contexts, and guidance warns that <10% is not automatically safe.
Do LEL values change with temperature?
Yes—many references note the flammability range expands as temperature increases (and often with pressure and enclosure size).